shares in a clinical-stage immunology-based biopharmaceutical company, RAPT Therapeutics, Inc. RAPT declined 12.07% yesterday after a 115, 5% had skyrocketed.
RPT193 is an investigational small molecule oral therapy for the treatment of atopic dermatitis and other inflammatory diseases. RPT193 was designed to selectively inhibit Th2 cell migration into inflamed tissues by blocking CCR4, a receptor that is highly expressed on Th2 cells.
The Phase Ib portion of the study is a randomized, double-blind, placebo-controlled study evaluating RPT193 as monotherapy in patients with moderate to severe AD. The primary endpoint of the study is safety.
Secondary and exploratory endpoints include pharmacokinetics, biomarkers, and clinical efficacy assessed by multiple measures including percent change in Eczema Area and Severity Index (EASI) score, the Validated Investigator Global Assessment (vIGA), and the Pruritis Numerical Rating Scale ( NRS).
The top-line results showed that after four weeks of treatment, patients with moderate to severe AD who received RPT193 showed a 36.3% improvement from baseline in the EASI score, a standard measure of disease severity, compared to 17.0% in the placebo group.
Importantly, patients in the RPT193 arm experienced continued improvement and further separation from placebo, with a 53.2% improvement in EASI at the six-week timepoint compared to 9.6% in the placebo group at the two weeks following the end of the treatment showed .
RPT193 was well tolerated with no serious side effects. The positive data supports the potential of RPT193 as a safe, once-daily oral treatment for patients with atopic dermatitis and may represent an attractive therapeutic alternative to injectable drugs.
Therefore, the company plans to advance RPT193 to a phase IIb study in atopic dermatitis and a phase IIa study in asthma.
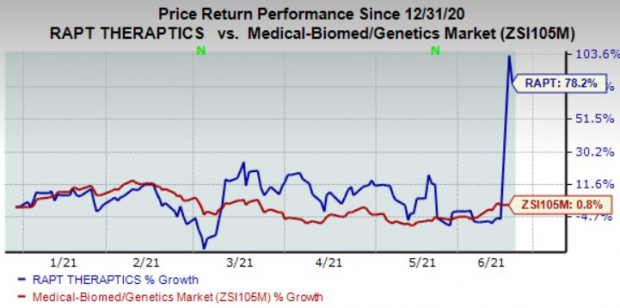
Shares of RAPT Therapeutics are up 78.2% year-to-date, compared to industry growth of 0.8%.
Image source: Zacks Investment Research
Sanofi SNY and regenerons (REGN Dupixent (Dupilumab) Injection is approved for the treatment of moderate to severe eczema (atopic dermatitis) in adults. Dupixent is intended for patients whose eczema is not adequately controlled by topical therapies or for whom topical therapies are inadvisable.
RAPT Therapeutics currently carries a Zacks Rank #4 (Sell).
A better ranked healthcare stock is Replicas Corporation RGEN carrying a Zacks Rank #2 (Buy). You can see the full list of today’s Zacks #1 Rank (Strong Buy) stocks can be found here.
Repligen’s 2021 earnings estimates have increased from $1.91 to $2.26 over the past 60 days.
Bitcoin, like the internet itself, could change everything
Blockchain and cryptocurrency have sparked one of the most exciting topics of discussion of a generation. Some call it the “Internet of Money” and predict it could change the way money works forever. If true, it could do to banks what Netflix did to Blockbuster and Amazon Sears. Experts agree that we are still in the early stages of this technology and as it grows it will create multiple investment opportunities.
Zacks just unveiled 3 companies that can help investors capture the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly lower volatility than buying them outright.
Check out 3 crypto-related stocks now >>
Want the latest recommendations from Zacks Investment Research? Today you can download the 7 best stocks for the next 30 days. Click here to get this free report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Research Report
Sanofi (SNY): Free Stock Research Report
Repligen Corporation (RGEN): Free Stock Research Report
RAPT THERAPTICS (RAPT): Free inventory analysis report
To read this article on Zacks.com, click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.